Lipid membrane destabilization induced by amyloid-beta peptide in the systems mimicking preclinical Alzheimer’s disease
1
Joint Institute for Nuclear Research, Dubna, Russia
2
Department of Biophysics, Dubna State University, Dubna,
Russia
3
Moscow Institute of Physics and Technology, Dolgoprudny,
Russia
4
Faculty of Pharmacy, Comenius University Bratislava, Bratislava,
Slovakia
Abstract
The amyloid-beta peptide (A\(\beta\) peptide) is proposed to play a central role in the onset of Alzheimer's disease (AD). The pathology is associated with the fast accumulation of neurotoxic amyloid aggregates in brain tissues, though the fundamentals of the disease's progression remain unsolved. It is noted that the preclinical stage of AD may play a crucial role in its further irreversible development. Namely, interactions between lipid membranes and A\(\beta\)-peptide molecules incorporated therein at relatively low concentrations should be under a close attention. In this review, we discuss recent works devoted to studying the lipid–peptide interactions with a specific focus on the lipid membrane reorganizations caused by A\(\beta\)(25–35) peptide in the preclinical AD mimicking conditions. The interactions observed are believed to be important in understanding the mechanisms of the A\(\beta\)-peptide destructive effects on lipid membranes and the corresponding onset of the disease. The methods of applied nuclear physics have proven remarkably relevant in such research. The scattering methods provided instrumental information on a level of supramolecular assemblies, while spectrometry allowed obtaining information on the molecular level. Finally, molecular dynamics simulations provided details unachievable by experimental approaches, though the validation role of the latter cannot be undermined. Altogether, the recent advances in research results prove these complementary approaches the most appropriate for tackling the complex issues of biomembrane interactions.
Keywords: Alzheimer’s disease, amyloid-beta peptide, lipid membrane, structure, dynamics
1. Amyloid hypothesis in Alzheimer’s disease
Alzheimer's disease (AD) is a devastating neurodegenerative brain pathology manifested by the loss of neuron cells. AD is becoming a major problem for public health, since the disease is known to be the most common cause of dementia characterized by speech and memory disorders, impairment of cognitive and physical abilities [1]. Since aging plays a considerable role in the development of AD, the disease is an inalienable attribute of all countries in the 21st century. AD will manifest itself in the future especially often due to an increase in the human average life expectancy. For example, it was estimated that about 24 million people in the world suffered from AD in 2005, which was less than 0.4% of the world's population [2], whereas this number will increase to 131 million people by 2050 (\(\sim 1.35%\) of the population estimated). Notwithstanding that AD was first discovered more than a century ago, in 1906, the foundations of the AD progression elude unambiguous explanations to the present days.
There are several hypotheses for the onset of AD, such as cholinergic hypothesis, tau hypothesis, and amyloid hypothesis, to name but those most accepted [3–5]. One of the most recognized scenarios is an amyloid cascade hypothesis introduced in 1991 [5,6]. The A\(\beta\) peptide in its two isoforms 1–40 and 1–42 is presumed among the most possible causes of AD. Specifically, A\(\beta\) peptides form extracellular oligomers leading further to irreversible accumulation of large aggregates in the form of insoluble amyloid fibrils and plaques, which have been the hallmarks of AD from its very discovery. Recent advancements in the cryo-electron microscopy allowed determining structures of A\(\beta\) fibrils at the atomistic and supramolecular levels [7–12]. The studies are unified by the conclusion that amyloid fibrils consist of large elongated and twisted stacks of A\(\beta\) peptide exhibiting cross-\(\beta\) sheet conformation. However, a comprehensive understanding of the relation between a structure of fibrils and their functional role in AD has not been fully achieved.
Neurotoxicity of A\(\beta\) peptides has been attributed not only to their fibrillar forms. In 1998, the A\(\beta\) oligomer hypothesis was proposed [13]. It is based on the discovery that insoluble amyloid oligomers consisting of A\(\beta\) peptides are central nervous system neurotoxins that destruct particular synapses. This causes a selective nerve cell death leading to the onset of AD. Notwithstanding that the oligomers usually escaped a main focus of clinical trials, which are aimed mostly at the attempts to combat formation of amyloid fibrils, it is accepted nowadays that the oligomers are the most neurotoxic A\(\beta\)-peptide form becoming thus a potential target in the fight against AD [14–16].
Further, many investigations of A\(\beta\) peptide, including those under the primary focus of this review, are aimed at the studies of monomeric form of the peptide in cell lipid membranes, rather than insoluble amyloid fibrils and oligomers. The approach is substantiated by the emergence of A\(\beta\) peptides in the lipid membranes of brain cells at the preclinical AD stage. Specifically, the A\(\beta\) peptides are known to be cleaved enzymatically (by means of \(\beta\)- and \(\gamma\)-secretase) from amyloid precursor protein (APP), which plays substantial physiological functions in the central nervous systems [17]. After the cleavage, A\(\beta\) peptides are able to accumulate and aggregate either in the membrane or outside lipid environment (Figure 1).
The A\(\beta\)(25–35) peptide fragment (residues 25–35) consisting of 11 amino acids was also identified to form neurotoxic amyloid oligomers and fibrils. This A\(\beta\) peptide has been found in the brain of patients who suffered from AD. It is believed to be a result of processing the full-length A\(\beta\) peptide by proteases [20]. The A\(\beta\)(25–35) and its aggregates were documented to damage neurons, inhibit DNA-dependent protein kinase activity, lead to transcription dysregulations and decrease Ca\(^{2+}\)-ATPase activity [20–25]. Moreover, this peptide fragment is a transmembrane segment of APP that initially interacts with a lipid membrane of cells [26]. The peptide being a part of APP contains hydrophilic residues situated in the extracellular environment (25–28), whereas the transmembrane part containing 29–35 residues is hydrophobic (Figure 2) [27]. Under in vitro conditions, the A\(\beta\)(25–35) peptides reproduce the main
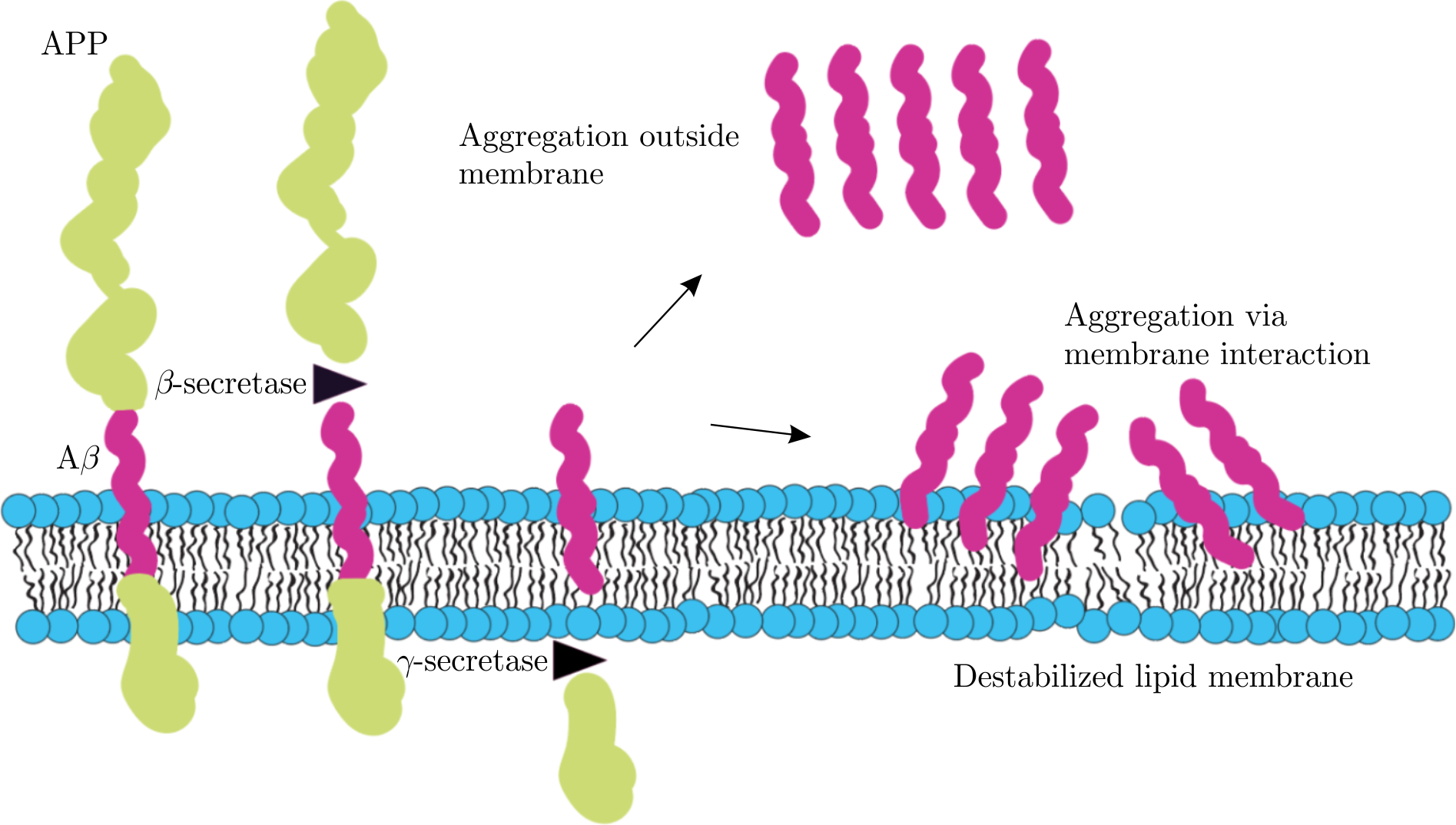
Figure 1. The sketch representing amyloidogenic cleavage of amyloid precursor protein (APP) into A\(\beta\) peptide by enzymes of \(\beta\)- and \(\gamma\)-secretase. The monomers of resulting A\(\beta\) peptides may be involved either in their aggregation in extracellular environment or in membrane destabilization upon their interactions directly with lipid membrane. Both ways lead to peptide cytotoxicity. Graphics is adapted from [18,19].
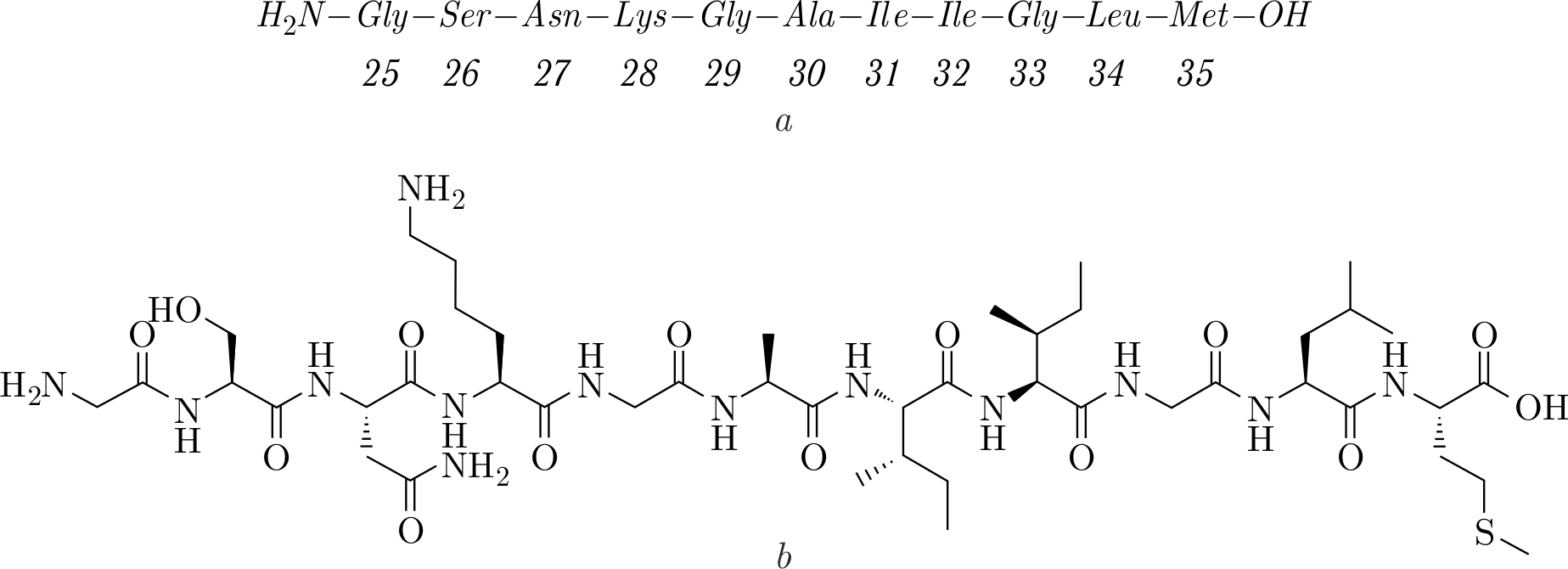
Figure 2. The nucleic acids sequence of the A\(\beta\)(25–35) peptide (\(a\)) and its chemical structure (\(b\)).
properties of full-length A\(\beta\) peptide. For example, molecules of A\(\beta\)(25–35) form aggregates that sediment in solution within several hours depending on the environment [28,29]. Thus, A\(\beta\)(25–35) may serve as a convenient tool to study molecular mechanisms causing AD.
The A\(\beta\)-peptide interactions with lipid membranes under in vitro conditions have been investigated quite extensively in recent years. Namely, researchers study the membrane structures, peptide conformations and peptide localization in membranes in connection with the development of many neurodegenerative diseases [30]. After all, the lipid bilayer as a main component of cell membranes plays an important role in the defense against the aggressive factors of outer environment and provides plenty of functions essential for cell activity. There are studies that indicate capability of A\(\beta\) peptides to damage lipid membranes via three mechanisms of action proposed as models of membrane destruction [31]. The mechanisms include a) membrane destabilization by prefibrillar peptide units (so-called carpet model), b) formation of transmembrane amyloid pores that can function as ion channels, and c) membrane damage by peptide monomers or oligomers that are able to extract lipids from the membrane (detergent-like interactions). All these processes violate the integrity of membranes and their normal function. Interestingly, there is evidence for the presence of lipids in amyloid fibrils found in brain tissues of patients with AD, suggesting a possible direct link between fibril growth and involvement of lipids [32]. Therefore, understanding the lipid–peptide interactions is a necessary step in the studies that reveal the role of the mentioned processes in the AD pathogenesis.
The lipid–peptide interactions depend on temperature that also defines a thermodynamic state of lipid membranes. In turn, various membrane-active molecules (such as cholesterol and melatonin), metal ions from intracellular and extracellular environment, and membrane proteins determine lipid–peptide interactions. Altogether, these factors affect structural organization of lipid membranes and regulate their dynamic properties [33–39]. Tuning up the structure and dynamics of lipid membranes by temperature and different biologically relevant additives is assumed one of the ways to prevent the neurotoxic effects of A\(\beta\) peptides on the lipid membrane.
Due to the compositional complexity of cell membranes, it is convenient to study artificially prepared model membrane systems, e.g., spherical lipid vesicles, which mimic the main distinctive features of the bilayered membrane structure [40]. Moreover, it is important to examine membranes that contain the various lipid species of interest in order to gain insights into the roles of individual lipid components in lipid–peptide interactions. The membrane composition of brain and its compartments is unique in a high concentration of total lipids (\(\approx \!50{\%}\) of the dry weight of brain is the lipid mass [41]) and in a content of lipid fractions. Among widespread lipids, there are phospholipids that include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), and cardiolipin [42]. Intriguingly, the overwhelming majority of all phospholipids in different brain compartments are represented by PC and PE [43].
PCs are well-known species of lipids ubiquitous in brain tissues and potentially participating in the onset of AD [43,44]. There are PC lipids differing by hydrocarbon chain length and the number of carbon double bonds, such as 1,2-dimyristoyl-sn-3-phosphatidylcholine (DMPC, 14:0), 1,2-dipalmitoyl-sn3-phosphatidylcholine (DPPC, 16:0), 1,2-palmitoyloleoyl-sn-3-phosphatidylcholine (POPC, 16:0-18:1), to name but those most relevant. DMPC and DPPC are fully saturated phospholipids, while POPC is a monounsaturated phospholipid. While brain phospholipids, when compared to other organs, are known to be highly enriched in polyunsaturated fatty acids, DPPC usually takes the first or second place in abundance amongst PCs [43–45]. The relative number of different types of lipids has been reported to change in brain tissues affected with AD [43–47]. Moreover, disturbances of the content of saturated lipids in brain were directly attributed to the risk of AD [47].
There are studies that propose considerable roles of lipid rafts in the AD development. The lipid rafts are mobile membrane microdomains that are highly enriched with cholesterol and sphingolipids, whereas differing from surrounding lipids [48]. The rafts form a liquid-ordered phase with lipids being more ordered and tightly packed. The heterogeneous separation of membranes provided by lipid rafts leads to changes in the dynamic and structural properties of lipids and surrounding membrane molecules. The importance of lipid rafts in A\(\beta\)-peptide aggregation, neurotoxicity, and the onset of AD has been reviewed recently[49–51]. For instance, more liquid-ordered rafts were found in patients with AD compared to those found in a control group. In addition, the observation brings attention to the variations in membrane lipid composition that can change with aging, thus linking it to the AD risk [52–54]. The lipid composition of membranes, along with their phase separation, is therefore an integral part of the amyloid hypothesis in AD, as it ultimately influences the pathways that promote the AD development as well as those with a normal function.
It is noteworthy that a close focus on A\(\beta\) peptides is also relevant from a therapeutical point of view. The molecular processes occurring within peptides under different conditions serve as a basis for the development of drugs that are introduced into clinical trials of patients suffering from AD. The trials are rich in the synthesis, modification, and adaptation of new promising anti-A\(\beta\) medicine aimed against formation of amyloid deposits[55]. However, the findings have not been completely translated to clinical practice because of prevailing inefficiency of the drugs designed. Nevertheless, some approaches for fighting the A\(\beta\) toxicity emerged in recent years. The examples include immunotherapy approaches, such as development of anti-A\(\beta\) monoclonal antibodies, adoptive therapy with A\(\beta\) specific regulatory T cells, and A\(\beta\) vaccines [56–59]. Intriguingly, a progress in nanotechnology also provides perspectives in designing nanoparticles that have a tunable chemical composition and strong specificity towards amyloid deposits. The nanoparticles are able to bind to peptides and therefore could be possible candidates for the treatment of the disease in the future [60,61].
2. Interactions of A\(\beta\)(25–35) with lipid membranes
The studies of lipid–peptide interactions are primarily the tasks of {\it in vitro} investigations of model lipid systems. In such approaches, low-resolution physical methods are indispensable. For instance, small-angle neutron and X-ray scattering (SANS and SAXS correspondingly) and diffraction, and solid-state nuclear magnetic resonance (NMR), Raman and circular dichroism (CD) spectroscopies are among the most informative research techniques [62] They serve as a foundation for solving internal and overall structures of lipid membranes and proteins at various scale levels. In addition, the limits of existing experimental approaches may often be extended further by computational methods. Molecular dynamics (MD) simulations are able to complement many new details to the overall picture of the lipid membrane structures and protein conformations.
The physical methods have been utilized in the studies examining the interactions of A\(\beta\) peptides with model membranes composed of various lipids. The translational dynamics of lipid membranes including DMPC/DMPS (DMPS — 1,2-dimyristoyl-sn-3-phosphatidylserine) mixtures was studied upon the addition of A\(\beta\)(25–35) to the oriented lipid membranes [63,64 ]. It was found that the A\(\beta\) peptides accelerate the lateral self-diffusion of lipids, which indicates that lipids are more disordered and destabilized in the presence of peptide molecules. Similar membrane-destabilizing behavior was also found for the full-length peptide A\(\beta\)(1–42). Namely, Dante and colleagues [65] studied the ability of A\(\beta\)(1–42) to interact with unilamellar vesicles whose membrane was formed by a mixture of POPC/POPS (1,2-palmitoyloleoyl-sn-3-phosphatidylserine). It was found that A\(\beta\)(1–42) was readily incorporated into the membrane, while disordering the lipids. At the same time, the dynamics of A\(\beta\)(25–35) molecules was studied during their interaction with micelles that served as model membranes. It is noted that the part of the A\(\beta\)(25–35) peptide containing residues 25–27 is highly ordered and less dynamic, while the region of residues 28–35 is disordered [66].
The localization of A\(\beta\)(25–35) peptide molecules in membranes is not always clearly defined and it strongly depends on many factors. They mostly include the lipid and charge composition of the membrane, external conditions, as well as sample preparation protocol. For example, Mason and colleagues [67] showed that A\(\beta\)(25–35) is mainly localized in the hydrophobic part of the membrane with a predominant predisposition of the charged N-terminus (25–28) near the head groups of phospholipids in a membrane composed of POPC. Similar results were obtained by Dante and coworkers for a POPC/POPS lipid mixture [68]. Also, it was revealed that the lipid composition mainly affects the localization of the hydrophobic part of the peptide. The C-terminal fragment of the peptide enters the membrane at different depths (6 Å or 14 Å from the center of the bilayer along the normal to the membrane in the case of a system without and with PSs, respectively) depending on the POPC/POPS lipid ratio. In addition, it was reported that the peptide strongly disorders lipids, increasing the membrane fluidity. Computer simulations [69] revealed two stable states in which A\(\beta\)(25–35) peptide can be found in the lipid membrane: near the surface of the DMPC lipid bilayer with a predominant inclination of the N-terminus inside the membrane, and a state in which the peptide is relatively deeply localized in the membrane. There are studies of electrostatic interactions of the A\(\beta\)(25–35) peptide with a membrane composed of a mixture of POPC/POPG (POPG — 1,2-palmitoyloleoyl-sn-3-phosphatidylglycerol) [70]. Namely, attraction of the positively charged part of the peptide to the lipid head groups of anionic lipids has been found. It allows the peptide molecules to be situated closer to the membrane–water interface. In the case of a mixture of saturated lipids (DMPC/DMPS), Dies et al. [71] found the A\(\beta\)(25–35) peptides being either incorporated into the membrane or in membrane-bound state. Ermilova et al. [72] reported that the degree of A\(\beta\)(25–35) incorporation into lipid bilayers depends on the saturation of lipid chains. The peptide is prone to situate near the hydrophilic region in the membrane made of unsaturated lipids, while it resides on the surface of the bilayer made of saturated phospholipids and tends to aggregate. The influence of lipid saturation on peptide localization is anticipated, since acyl chain unsaturation perturbs hydrocarbon chain region, resulting in increased chain disorder. Lau and colleagues [73] concluded that the localization of A\(\beta\)(25–35) peptide in the membrane also depends on the sample preparation procedure. While A\(\beta\)(25–35) molecules reside closer to the hydrophobic part of the lipid bilayer in the case of the membrane that contains peptides incorporated during the sample preparation, the peptides are prone to adsorb onto membrane surface in the case of the peptide addition to the solution of vesicles. Also, A\(\beta\)(25–35) molecules were reported to demonstrate spontaneous membrane insertion from water environment, thus confirming the ability of A\(\beta\)(25–35) to strongly interact with lipid bilayers [74]. Consequently, despite the fact that many studies have revealed two preferred localizations of the A\(\beta\)(25–35) peptide in membranes, the location of the peptide is hard to predict for a particular system.
There are observations of a tendency of A\(\beta\)(25–35) peptides to aggregate in lipid membranes. It has been suggested that the A\(\beta\)(25–35)–membrane interactions occur in three stages: adsorption of peptides from surroundings, nucleation of aggregates, and their penetration into membranes [75]. In addition, it was shown that the monomeric form of the peptide has a trend to form insoluble large structures in DMPC and DOPC (1,2-dioleoyl-sn-3-phosphatidylcholine) lipid bilayers [76]. Some data described accelerated formation of peptide clusters representing \(\beta\)-sheet secondary structures inside membranes consisting of a mixture of POPC and DMPC (or DMPS) lipids [77,78]. The number of aggregates significantly increased at relatively high concentrations of the peptide (\>10\) mol%, where mol% is the peptide/lipid molar fraction), while the size of the clusters did not depend on its amount. A characteristic feature of such aggregates was a significant discrepancy between the hydrophobic thicknesses of peptides and the lipid bilayers that led to local distortions of the membrane structure and disordering of lipids near the peptides. The studies on A\(\beta\)(25–35)–membrane interactions have also shown ion-conducting pore formation by the peptide [79,80]. While some results proposed a model of membrane pores formed by A\(\beta\)(25–35) peptides having \(\alpha\)-helical secondary structure [81], other data revealed that the A\(\beta\)(25–35) peptide incorporated into membrane forms 6- or 8-stranded \(\beta\)-barrel-like structures [82]. The \(\beta\)-barrel-like structure provides an inner cavity of 6–7 Å radius, where A\(\beta\)(25–35) secondary structures are represented significantly by \(\beta\) sheets that penetrate the lipid membrane consisting of POPC/POPG. These channels conduct calcium ions that can disrupt their homeostasis, and therefore they are considered toxic.
3. Morphological reorganization of lipid membranes triggered by A\(\beta\)(25–35)
Although a significant amount of literature is dedicated to the studies of interactions between A\(\beta\) peptides and lipid membranes, it is important to scrutinize the general structural organization of lipid membranes in solution when interacting with A\(\beta\)(25–35) peptides. SAXS and SANS may come to the rescue providing valuable insights into shapes of lipid aggregates (i.e., their morphology, such as a sphere or a disc) and revealing the structural parameters of lipid bilayers. The methods provide small-angle scattering curves that depict intensities \(I\) of a scattered beam as a function of scattering vector \(q\). The resulted curves may be fitted by using model-based approaches to extract quantitative values of membrane structural parameters. The length scales probed by small-angle scattering methods range from 1 to over 100 nm, which is relevant to the dimensions of both the membrane and overall vesicle. Therefore, the vesicle radius (\(R\)) and membrane thickness (\(d_{L}\) or \(d_{HH}\) obtained from SANS and SAXS, respectively [83]) as the most useful parameters describing the overall structure of lipid objects may be calculated from the analysis of small-angle scattering curves. The information about vesicle size is contained in the low-\(q\) region of the curves, where the scattering is sensitive to large length scales (i.e., according to the relation \(q = 2\pi/d\), where \(d\) is a characteristic dimension), while the information about bilayer structure lies in the mid- and high-\(q\) regions.
SANS and SAXS methods have detected the changes in membrane morphology in the lipid vesicles composed of saturated DPPC or DMPC lipids and A\(\beta\)(25–35) peptides incorporated into membranes at relatively low peptide concentrations (\(<\!5\) mol%). Such systems mimic the preclinical AD stage. Namely, a reorganization of unilamellar vesicles (ULVs) into discoidal structures and small unilamellar vesicles (SUVs) has been observed (Figure 3a) thanks to distinct features of the SANS (and SAXS) curves in the low-\(q\) region in the case of different lipid morphologies (ULVs, discs and SUVs) (Figure 3b) [84]. Initial ULVs were obtained via an extrusion through polycarbonate filters with pores of 500 Å diameter [85,86]. The ULV radius was \(\approx 350\) Å [84]. The radii of emerging discs and SUVs were estimated to be around 200 and 150 Å, respectively. Such reorganizations of membrane general shape happened during the main phase transition temperature (\(T_{m}\)) of lipids when heating and cooling the samples through \(T_{m}\) and only in the presence of the A\(\beta\)(25–35) peptides embedded in the lipid bilayer. Along with transformations in membrane morphology, parallel changes in the membrane thickness have been detected. The changes were comparable to those observed upon phase transitions of lipid membranes (without A\(\beta\)(25–35)), thus confirming the phase transition of membranes in the presence of A\(\beta\)(25–35)[84].
The abrupt changes in the membrane morphologies when crossing \(T_{m}\) were interpreted as an A\(\beta\)(25–35)-triggered destabilization of membranes and a consequent reorganization of their supramolecular structure [84]. It should be emphasized that lipid membranes do not get damaged, rather they break and convert their shape between states stable at different thermodynamic phases. Reorganizations between different lipid morphologies (e.g., disc–vesicle or disc–micelle transitions) are known to be observed in mixtures of short-chain and long-chain
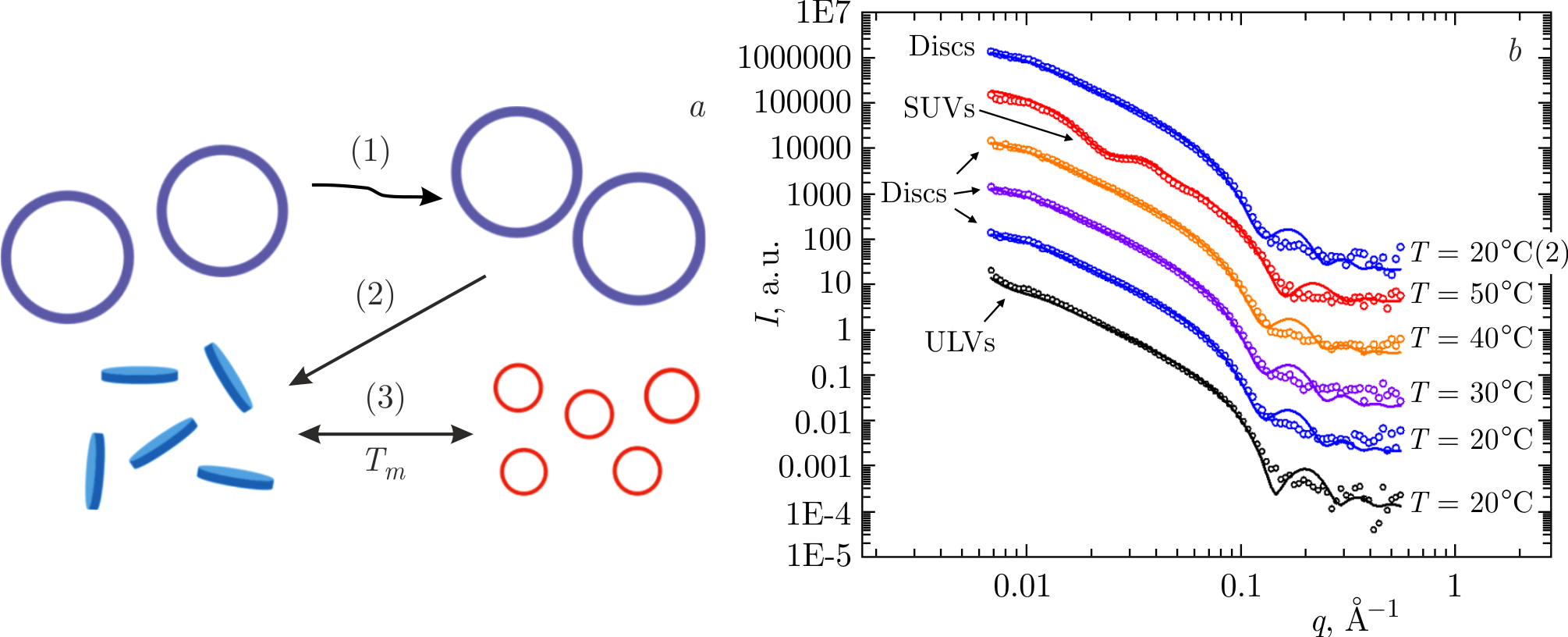
Figure 3. \(a\)) Sketch of morphological reorganizations of lipid membranes consisting of saturated lipids (DPPC or DMPC) triggered by A\(\beta\)(25–35) peptide monomers incorporated into lipid bilayers. Having been unaffected upon heating from the gel phase to the fluid phase through the main phase transition temperature (\(T_{m}\)) of lipids (1), unilamellar vesicles (ULVs) undergo an irreversible reorganization to discs upon subsequent cooling (2). Upon further heating and cooling through \(T_{m}\), there are reversible reorganizations between the discs (below \(T_{m}\)) and small unilamellar vesicles (SUVs) (above \(T_{m})\) (3). \(b\)) SANS curves (symbols) and their best fits (lines) for DPPC\(+\)A\(\beta\)(25–35) samples in the temperature range of 20–50\(^{\circ}\)C. The small-\(q\) region of the curves displays the features inherent to different morphologies of the lipid objects investigated (ULVs, discs, or SUVs). The figure is adapted from [87].
lipids [88,89], upon addition of various surfactants, detergents [90–93, and peptides [94–99, such as melittin — a membrane lytic peptide. In most cases, the effects of reorganizations occur near \(T_{m}\) and are known to be dependent on molecular composition and outer environment, whereas the peptides themselves are frequently capable of disrupting lipid membranes. Several mechanisms of antimicrobial peptide actions have been proposed, including formation of toroidal pores penetrating a membrane and extraction of lipids from a membrane by peptide molecules [100]. It has also been presumed that A\(\beta\) and antimicrobial peptides share similar molecular mechanisms leading to lipid membrane destabilizations [101,102].
The changes in the membrane structure and dynamics during the phase transition may be a foundation to a temporary membrane breakage. A typical feature of the phase transition of lipid membranes containing a single type of saturated lipids is a sharp jump in membrane characteristics. It is indicated by a change in the lateral lipid diffusion rate, ordering of lipid hydrocarbon chains, head group hydration, and intermolecular entropy [103]. Membrane thermodynamic phase transitions upon gradual heating include the lipid pretransition (identified by pretransition temperature \(T_{p}\)) from the gel to ripple phase with subsequent main transition (\(T_{m}\)) to the fluid phase [104,105]. The gel phase of membranes is distinguished by highly ordered lipids, in contrary to the disordered fluid state [104]. The ripple phase is known to form sawtooth-like membrane surface with alternation of two areas containing more ordered and disordered lipids [106,107], though the recent studies suggested more complex ripple phase structure involving several different lipid conformations [108,109]. During the phase transitions upon heating, a lipid membrane switches between the less diffusive form and the more diffusive fluid state that may influence lipid–peptide interactions, including those responsible for membrane destabilization.
In addition to the lipid structural properties, an A\(\beta\)-peptide secondary structure, which is also known to vary significantly in different membrane thermodynamic phases, may contribute to the membrane destabilization. It was found that A\(\beta\) peptides undertake \(\alpha\)-helix, random coil, and \(\beta\)-sheet structures in different membrane environments. For instance, Yoda et al. previously reported that full-length A\(\beta\)-peptide secondary structures were able to change between all the mentioned types, when the lipid membrane was crossing various thermodynamic states during its heating [110].
The transition of A\(\beta\) peptides from more disordered random coils to ordered \(\alpha\)-helices was concluded to be important in the case of fibril formation, since the emergence of \(\alpha\)-helices have been considered as an intermediate key step in the formation of A\(\beta\) fibrils containing \(\beta\) sheets [111,112]. It is known that not only a full-length A\(\beta\) peptide but its fragments, A\(\beta\)(25–35) in particular, may adopt \(\alpha\)-helix in the presence of a zwitterionic lipid bilayer [113–115]. Such transitions are apparently caused by the hydrophobic interactions between A\(\beta\)-peptide molecules and lipid chains [116]. On the one hand, the emergence of \(\beta\)-sheet conformations of A\(\beta\) peptides is associated with fibrillogenesis [117]. On the other hand, the lipid environment is known to affect the secondary structure of membrane-active peptides that eventually leads to the disruptions of the bilayer integrity [118].
Given a crucial contribution of A\(\beta\)(25–35) peptides to the membrane destabilization when crossing \(T_{m}\), the secondary structures of A\(\beta\)(25–35) peptides incorporated in the discs and SUVs have been an object of close attention. Recent results obtained by using circular dichroism spectroscopy, Raman spectroscopy, and molecular dynamics simulations displayed secondary structures represented predominantly by random coils (\(\approx\!\!50\)%), while changing little when reorganizing between discs and SUVs (being in the gel and fluid phase, respectively) [87]. The data indicated that the secondary structure of A\(\beta\)(25–35) does not influence the morphology of the lipid objects, just as the thermodynamic state of the lipid membrane does not affect the A\(\beta\)(25–35) secondary structure.
Notwithstanding neuronal toxicity of A\(\beta\) peptides, it is known that A\(\beta\) peptide may participate in important physiological functions. There are studies reviewed in [119] that describe possible roles of A\(\beta\) peptides in protection against some forms of cancer, regulation of activity at hippocampal synapses, and emergence of antimicrobial properties. It is worth noting that the peptides should only be regarded as toxic when their production is excessive, and aggregation is imbalanced. While normal A\(\beta\)-peptide concentration range lies in picomolar and nanomolar intervals[120,121], elevated peptide concentrations were reported to cause, for example, a rapid A\(\beta\)-peptide aggregation into cross-\(\beta\) sheets, which strongly disturb the lipid bilayer [77]. Therefore, the effect of higher A\(\beta\)-peptide concentrations on the morphological reorganizations from ULVs into discs and SUVs has also been scrutinized [87]. The effect has been studied in the extended concentration range of the micromolar (relative to water) interval. Interestingly, but not unexpectedly, morphological reorganizations were observed only above a specific concentration of A\(\beta\)(25–35) molecules (\(\geqslant\!1\) mol%) incorporated into the lipid membranes [87]. Namely, the ULVs containing various concentrations of A\(\beta\)(25–35) peptides incorporated in DPPC membrane were subjected to heating–cooling cycles in order to launch the chain of A\(\beta\)(25–35)-driven morphological reorganization. Comparing the values of radius\(R\) of initial ULVs (Figure 3, empty symbols) obtained by SAXS measurements with those of the final-state samples (ULVs or discs) after heating–cooling cycles (Figure 4, full dots), one may notice a critical peptide concentration, above which morphological reorganization is observed. This effect may be well related to the recognized AD pathogenesis that resulted from an excessive accumulation of A\(\beta\) peptides in brain tissues of AD patients. Further, the importance of
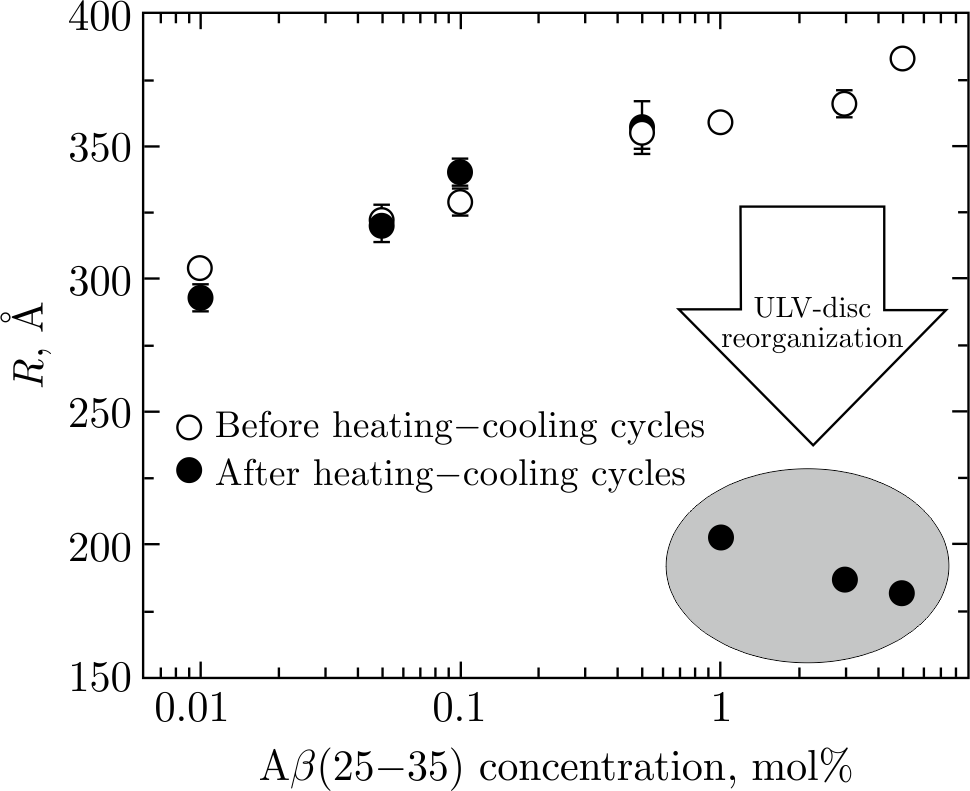
Figure 4. Radii (\(R\)) of ULVs at 0.01–0.5 mol% of A\(\beta\)(25–35) and radii of discs at 1–5 mol% of A\(\beta\)(25–35) in DPPC\(+\)A\(\beta\)(25–35) systems after heating–cooling cycles of ULVs (full dots). For the clarity of presentation, empty dots display the same samples before heating–cooling cycles. All \(R\) values were obtained at \(T = 20^{\circ}\)C in the DPPC gel phase. The drop of \(R\) values at 1–5 mol% of A\(\beta\)(25–35) indicates the ULVs-to-discs reorganization after heating–cooling cycles performed on initial ULVs. The circled group of points describes the \(R\) values of discoidal objects. The results were taken from [87].
critical concentration of A\(\beta\) peptides was emphasized by in vitro experiments, when increasing the concentrations of full-length A\(\beta\) peptides in lipid supported bilayers. The peptides led to the disruption of lipid membranes via lipid extraction and formation of holes [122,123]. Hence, the morphological reorganization of ULVs that happens when crossing \(T_{m}\) is a direct evidence of peptide–lipid interactions playing a key role in the membrane breakage when reaching a certain peptide concentration.
4. Lipid–peptide arrangements in discoidal structures and unilamellar vesicles
When searching for the mechanisms of the lipid membrane reorganization triggered by the disruptive effects of A\(\beta\)(25–35) peptides, it is increasingly important to study the lipid–peptide arrangements in the membrane objects formed. A wide variety of membrane proteins and peptides induce membrane curvature and change their closest lipid environment for a specific purpose [124,125]. For example, clathrin, a membrane protein involved in the transport processes of substances, performs an important function by concentrating epsins that contribute to membrane deformations and subsequent emergence of separate transport vesicles [126]. The induction of a membrane curvature is also an example of the damaging impact of amyloidogenic proteins on membranes [127]. The A\(\beta\)(25–35) peptide and lipid co-localization in lipid membranes may prove to represent a key factor in the mechanism of morphological reorganization induced by A\(\beta\)(25–35).
Solid-state \(^{31}\)P NMR spectroscopy and coarse-grained molecular dynamics (CG MD) simulations provided an excellent basis to study the overall lipid–protein arrangement, including a specific A\(\beta\)(25–35) peptide localization in the lipid membranes of discs and SUVs [128]. The \(^{31}\)P NMR spectroscopy detects a signal from phosphorus nuclei comprised in the phosphate group of lipids and reveals changes in the chemical shift. Chemical shift anisotropy of the \(^{31}\)P nucleus is relatively high, which allows studying lipid membrane structures, various lipid phases and membrane thermodynamic states [129,130]. It was shown that disc-forming lipid membranes with incorporated A\(\beta\)(25–35) demonstrate magnetic alignment in the NMR spectrometer, resulting in the emergence of sample's anisotropy (Figure 5) [128]. Namely, the non-zero resonance lines (anisotropic phase of the sample) in the \(^{31}\)P NMR spectra are associated with magnetic alignment of discs oriented with the normal of the lipid bilayer perpendicular to the direction of magnetic field, whereas the emergence of the isotropic phase (at 0 ppm) may be attributed to SUVs. Upon heating, the increasing alignment of discs improves disclosing the well-resolved lines, thanks to which it is possible to unravel the lipid arrangement of the discs. Two pronounced peaks corresponding to the anisotropic phase are attributed to the lipids located in the flat bilayered part of the disc (\(\approx\!-13\) ppm) and those that cover the round disc edge (\(\approx\!-9\) ppm).
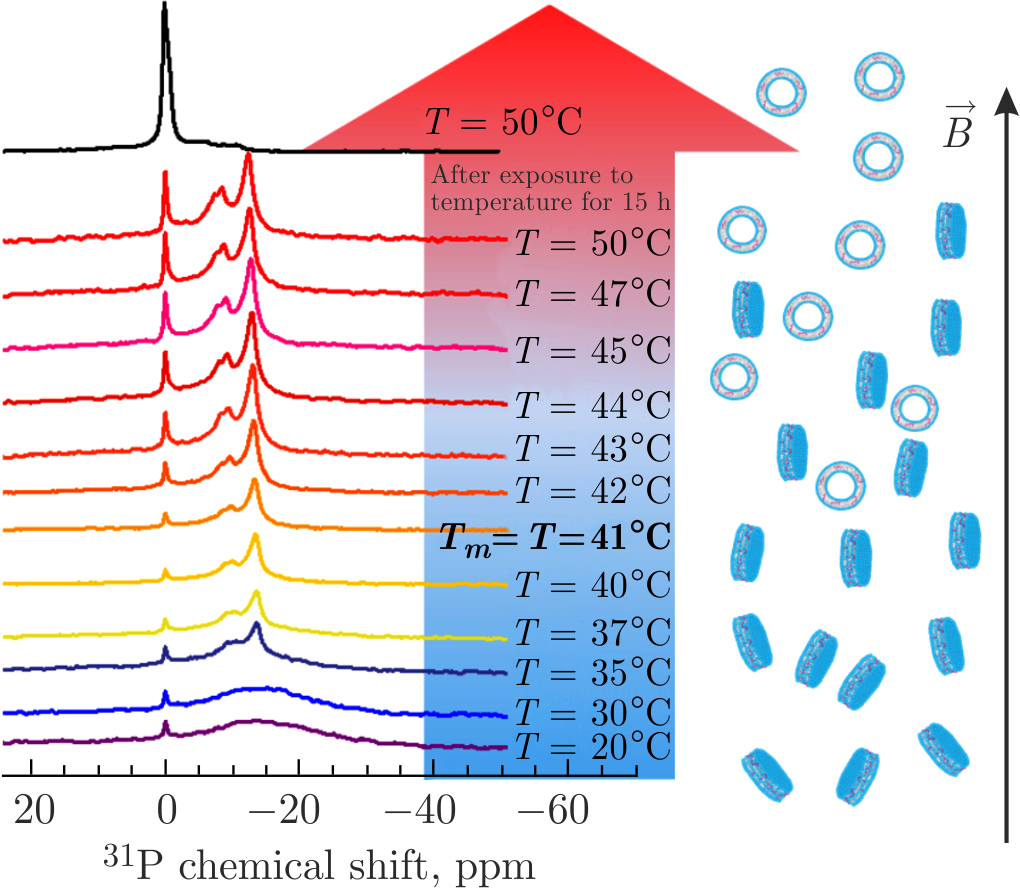
Figure 5. The \(^{31}\)P NMR spectra of DPPC\(+\)A\(\beta\)(25–35) systems recorded upon gradual heating through \(T_{m}\) of DPPC lipids with subsequent sample equilibration at \(T = 50^{\circ}\)C for 15 h in NMR spectrometer. The isotropic phase at 0 ppm corresponds to SUVs undergoing fast isotropic motions, whereas the anisotropic phase at non-zero resonances is associated with magnetically aligned discs. Adapted from [128].
It is worth noting that there are conventionally two types of discoidal membrane nanoobjects discussed in literature: bicelles [88,131,132] and nanodiscs [133–135]. Being known for the ability to orient in magnetic fields because of their negative anisotropy of magnetic susceptibility, bicelles and nanodiscs are very important tools to study the lipid membrane and protein properties in a wide range of various external conditions [136,137]. A bicelle usually consists of a mixture of short-chain and long-chain lipids, where short-chain lipids construct half-torus of a discoidal structure and cover the hydrophobic part of a lipid bilayer consisting of long-chain lipids. An alternative to bicelles is provided by nanodiscs where the half-torus is composed exclusively of a long protein or polymer that emerges in a form of belt around the discoidal structure comprising single-type lipids.
The presented NMR spectra corresponding to the anisotropic phase are incompatible with those of nanodiscs oriented in a magnetic field, but they are typical to the NMR spectra of aligned bicelles. However, neither of the arrangements is supposed to apply directly to the system of discoidal objects that emerged as a result of A\(\beta\)(25–35)-triggered membrane destabilization, since they include only single-type lipids and short peptides. Due to this composition, they may be described as systems similar structurally to bicelles (i.e., bicelle-like structures — BLSs), in which lipids of the same type are situated both in the bilayered part and at the edges of BLSs. This would however represent an unstable combination. The explanation of the stable discoidal shape then lays in a specific lipid–peptide arrangement at the edges of BLSs. The perimeter of BLSs consists of the lipid–peptide mixture that prevents the interaction of water molecules with the membrane hydrophobic part (Figure 6a) [128].
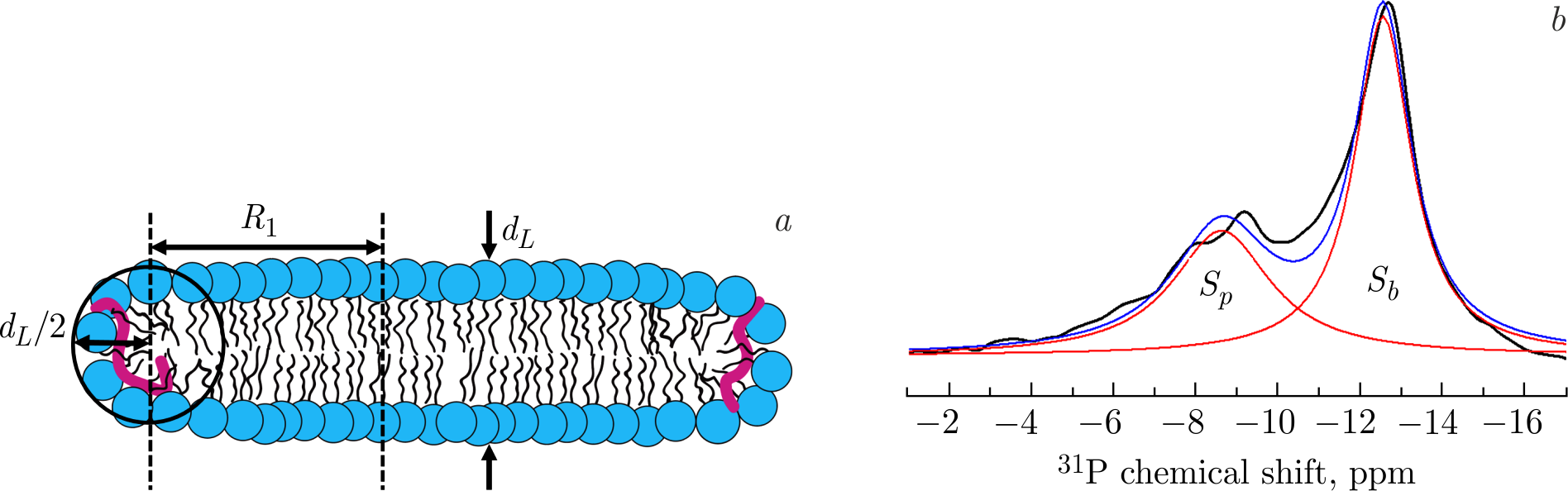
Figure 6. \(a\)) The 2D sketch displaying geometrical parameters of the BLS in cross-section and the lipid–peptide arrangement of the BLS. Lipid head groups are shown in blue, lipid chains are displayed in black, whereas the A\(\beta\)(25–35) molecules are displayed in red. \(b\)) An experimental \(^{31}\)P NMR spectrum (black line) of magnetically aligned BLSs at \(41^{\circ}\)C [128]. The spectrum is approximated by two Lorentzian functions (red lines); blue line corresponds to the sum of the two Lorentzian functions.
Based on the presented \(^{31}\)P NMR spectra, it is possible to obtain structural parameters of the BLSs, their characteristic sizes in particular. The integral value of intensities of the \(^{31}\)P resonances corresponding to anisotropic phase of the sample reflects the ratio of the number of lipids situated in the bilayered part of BLSs (\(N_{b}\)) to that in the perimeter of BLSs (\(N_{p}\)). Given the ratio of peak intensities, it is possible to calculate the disk diameter. \(N_{b}\) and \(N_{p}\) are proportional to the surface area of the BLS and the area of the edges, respectively. While the surface area of BLS's flat part (\(S_{b}\)) represents two lipid monolayers: \(S_{b}=N_{b}A_{L}=2\pi R_{1}^{2}\), the surface area of BLS's perimeter (\(S_{p}\)) can be approximately estimated as the surface area of half-torus: \(S_{p}=N_{p}A_{L}=2\pi R_{1}\displaystyle\frac{\pi d_{L}}{2} = \pi^{2}R_{1}d_{L}\), where \(d_{L}\) is the thickness of the DPPC lipid bilayer, \(R=R_{1} + \displaystyle\frac{d_{L}}{2}\) is the radius of BLS including lipids located on a half-torus, \(A_{L}\) is the area per lipid (see Figure 6a for the sketch). Assuming that the DPPC lipids on the BLS surface and the BLS edges have the same average \(A_{L}\), the BLS's diameter (\(D = 2R\)) is \(D = \displaystyle\frac{S_{b}}{S_{p}}\pi d_{L}+d_{L}\). The \(S_{b}/S_{p}\) ratio reflects the relative areas under the peaks in the \(^{31}\)P NMR spectra evaluated by fitting (Figure 6b). Thus, considering the characteristic bilayer thickness \(d_{L} \approx50\) Å of the gel phase DPPC membrane [83], the calculated diameter \(D\) of BLS is \(\approx\!400\) Å. This result is in an excellent agreement with SANS and SAXS data [84].
The NMR data may be successfully complemented by the results of CG MD simulations to obtain directly the A\(\beta\)(25–35) peptide distribution in membranes. In recent years, CG models have been able to provide approaches that allow a significant increase in the timescale of simulations [138]. Due to the grouping of individual neighboring atoms into larger units, it is possible to effectively model proteins and large lipid objects on the timescales hardly available to full-atom simulations[139]. First, it was shown by means of CG MD that the randomly distributed lipids, A\(\beta\)(25–35) peptides, and water molecules have self-assembled spontaneously into BLSs below \(T_{m}\) and SUVs above \(T_{m}\) after microsecond timescale simulations (Figure 7) [128]. The size of BLSs and SUVs was in a good agreement with that calculated from the SANS, SAXS, and NMR experiments. Second, the round edge of the BLSs was revealed to contain not only the lipid molecules but also the A\(\beta\)(25–35) peptides, which were situated at the perimeter of BLSs predominantly. The peptide–lipid co-localization at the edge of BLS allows this peculiar shape to be maintained in the aqueous environment. Thus, one of the possible disruptive mechanisms of the A\(\beta\)(25–35) peptide may be based on the ability of A\(\beta\)(25–35) to rearrange the lipid molecules around itself, creating locally a highly curved lipid bilayer.
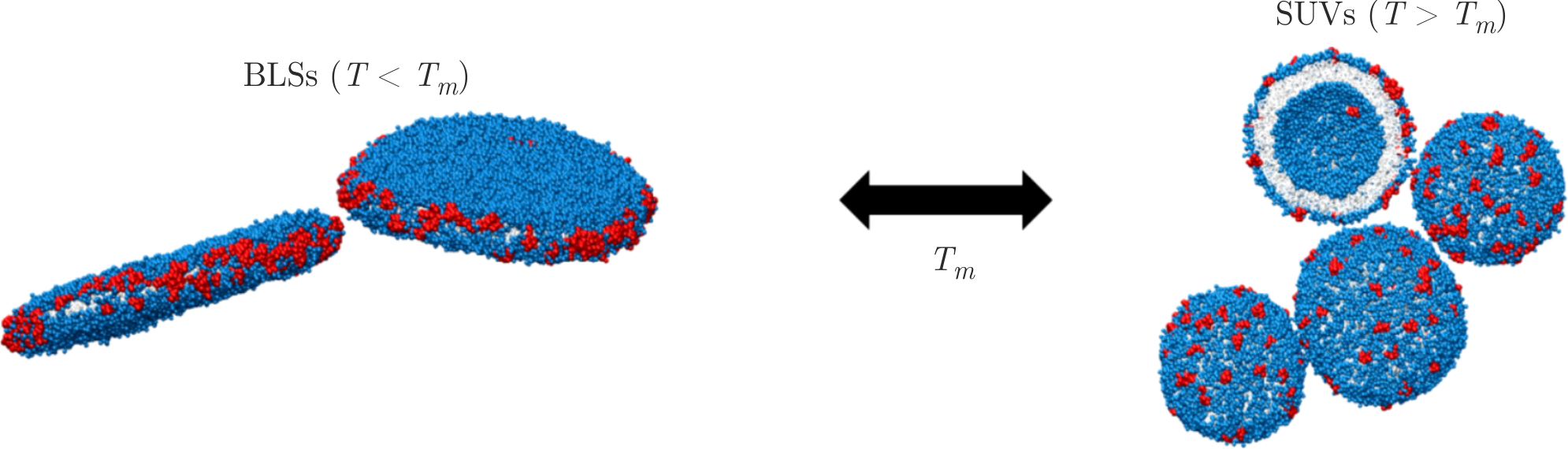
Figure 7. Snapshots of the CG MD simulations revealing the lipid–peptide arrangement in BLSs and SUVs, where one of the vesicles is depicted with its far part sliced through its central plane. Grey color corresponds to the lipid chains, lipid head groups are shown in blue, and A\(\beta\)(25–35) molecules are displayed in red. For the sake of better visualization, water molecules are not represented. Adapted from [128].
In the formed SUVs, A\(\beta\)(25–35) peptides appear to mix randomly with lipids within the bilayer and interact with the hydrophilic part and hydrophobic region of the membrane, as it was shown by CG MD [128]. The arrangement of SUVs reveals high curvature of the lipid bilayer with an asymmetric A\(\beta\)(25–35) peptide distribution between the outer and inner leaflets (see Figure 7). This effect is likely due to the less dense packing of lipids in the outer leaflet, which provides more space for localization of peptide molecules. The lipid head groups of the outer leaflet in highly curved membranes are known to be packed more freely compared to the head groups of the inner leaflet [140]. The A\(\beta\)(25–35) peptides are then prone to diffuse to the regions of lipid bilayers with relatively loose spacing. The data presented in this part of the review provide valuable information particularly about the distribution of A\(\beta\)(25–35) peptides within the highly curved membranes, as well as the A\(\beta\)(25–35) ability to bend the lipid bilayer and thus the reasons for stability of BLSs in water environment.
5. Effect of additives
Regulations of many cellular processes are known to be affected by the membrane composition and molecular surroundings, which may govern the fluidity and rigidity of membranes, their structural organization, and lipid–peptide interactions. However, the changes of membrane properties may result in dysfunction of mechanisms that regulate the vital activity of cells. As the onset of AD is primarily related to the peptide accumulation and elevated peptide concentrations, it is important to understand the effect of A\(\beta\)(25–35) on the membrane fluidity and vice versa. It turned out that the structure of a lipid membrane at the molecular level is greatly influenced by A\(\beta\)(25–35) peptides incorporated in membranes of ULVs. Namely, the increase in the membrane thickness with parallel increase in the ULV size has been detected [87]. The data indicate that A\(\beta\)(25–35) molecules promote an elevated membrane rigidity. In order to control the rigidity of lipid membranes with incorporated A\(\beta\)(25–35), some widespread membrane-active additives may be used, e.g., cholesterol and melatonin molecules, or calcium ions. Introduction of charged PS lipids existing at a certain fraction in the cell membranes is also an important factor for controlling the charge within the membrane.
Melatonin is a neurohormone produced in central nervous system. It has various functions, such as regulations of a circadian rhythm, energy metabolism, and inhibition of molecular oxidation [141,142]. Regarding AD pathogenesis, melatonin has been suggested to have a protective role against amyloid toxicity [143,144] due to their ability to inhibit A\(\beta\)-peptide production and formation of amyloid fibrils. While concentration of melatonin decreases during aging, especially for patients suffering from AD, the data from clinical trials have documented that melatonin supplementations inhibit cognitive impairment in AD patients [141,145].
Cholesterol is a major lipid constituent of most biological membranes reaching at least 52 mol% in myelin membranes [146]. It supports their integrity and serves as a precursor for the synthesis of several vital substances [147,148]. Cholesterol molecules present in neuronal membranes are able to regulate various protein functions. Several studies have connected an increased risk of the onset of AD and an elevated level of cholesterol concentration [149–151], while the correlation details still remain unclear [152]. Although cholesterol has been shown to play protective roles against amyloid toxicity, such as decreased A\(\beta\)-peptide accumulation and reduced A\(\beta\) tendency to assemble into membrane calcium ion channels [153,154], it has also been documented to support transmembrane pore formation along with increased peptide ability to incorporate into lipid membranes and bind to the membrane surface [51,155,156]. Interestingly, dual effect of cholesterol on the A\(\beta\)(25–35)–membrane interactions has been described: at relatively low cholesterol concentrations, the deep incorporation of the peptide is favored, while the cholesterol-rich membrane prevents the A\(\beta\)(25–35) insertion [157]. At the same time, A\(\beta\)(25–35) were found to displace cholesterol molecules into plaques lowering the cholesterol concentration in a lipid bilayer [71]. Melatonin molecules were concluded to decrease the fraction of membrane-incorporated A\(\beta\)(25–35) and to increase the amount of membrane-bound A\(\beta\)(25–35) [71], which can be explained by entropic effects due to increased membrane fluidity.
Melatonin and cholesterol are able to penetrate lipid membranes and regulate their structural and dynamic properties [36,158–161]: cholesterol is known to increase the thickness of membranes and reduce lipid lateral diffusion by increasing membrane rigidity, while the addition of melatonin to membranes results in the opposite effect. The impact of these molecules can be explained in terms of their different localization in the lipid bilayer. Namely, the cholesterol molecule having a small hydrophilic head and a hydrophobic tail is prone to be situated within the hydrocarbon chain zone of membrane, aligning itself parallel to the normal of membrane surface. Hydrophilic melatonin molecule, on the contrary, resides readily in the lipid head group region [35]. The peptide localization in the presence of cholesterol and melatonin molecules is demonstrated by MD snapshots displayed in Figure 8a and Figure 8b, respectively. A more complex construction presented in Figure 8c includes the A\(\beta\)(25–35)-containing membrane loaded with both cholesterol and melatonin molecules. The system illustrates the competitive effects (membrane rigidification documented by thickness increase vs. membrane fluidization documented by thickness decrease) of cholesterol and melatonin on the structural properties of lipid membranes with A\(\beta\)(25–35) incorporated [162]. The rigidifying effect of cholesterol on the membranes with or without peptide appears to be prevailing over the fluidizing effect of melatonin [36]. Also, the immanent localization of cholesterol and melatonin molecules is unaltered in this multicomponent system when compared to the separate systems. In addition, A\(\beta\)(25–35) peptides assume a combined localization within the hydrophobic part of bilayer, while preserving their interactions with both cholesterol and melatonin.
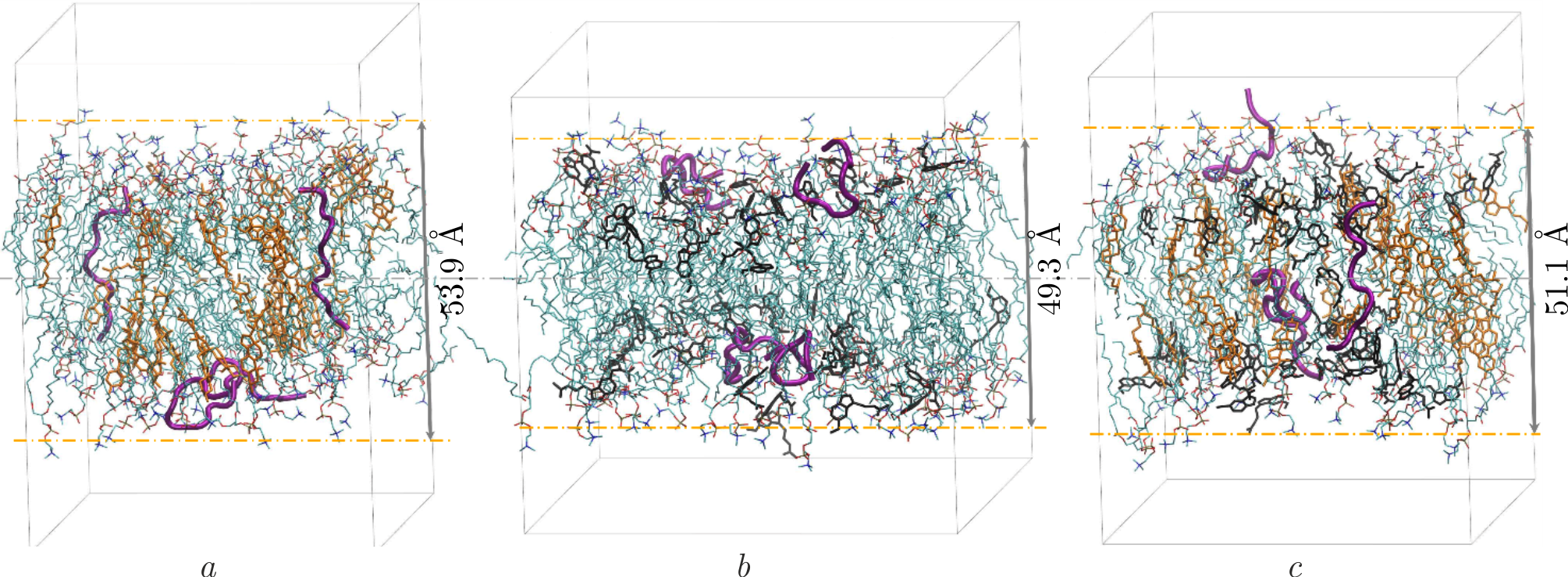
Figure 8. The snapshots of 100 ns MD simulations depicting the patch of DOPC lipid bilayer with initially incorporated A\(\beta\)(25–35) molecules (3 mol%) and loaded with \(a\)) cholesterol, \(b\)) melatonin, \(c\)) cholesterol and melatonin. Concentration of cholesterol and melatonin equals 29 mol%. Cholesterol molecules are shown in yellow, melatonin molecules in black, A\(\beta\)(25–35) peptides in pink, while lipids are represented in cyan. The numbers describe the lipid membrane thicknesses extracted from MD simulations in accordance with [162].
The electrostatic interactions are yet another factor crucial for vital activity of a cell. The important roles of such interactions in a whole range of physiological processes at the membrane–water interface and in the closest membrane surroundings cannot be overestimated. An essential role in regulating the functions and elasto-mechanical properties of membranes is therefore given to the charge as well, whether in the form of charged lipids included in the cell membranes or various ions omnipresent in outer environment. Calcium ions, whose concentrations amongst cell organelles varies from nanomolar to millimolar ranges and reaches 2 mM in extracellular space, take part directly in the mineralization and contraction of tissues, signal transduction, and protein synthesis [163–165]. The ions bind to lipid head groups, predominantly situating near phosphate groups and affect membrane structure, dynamic properties of lipids, and protein–lipid interactions [38,166–171]. Elevated concentrations of calcium ions have also been found near the deposits of A\(\beta\) peptides in AD affected brain tissues, suggesting possibly an essential role of the ions in the onset of AD [172,173]. There are studies revealing the ability of calcium ions to promote and accelerate formation of amyloid oligomers and fibrils [174,175], including aggregation of A\(\beta\) peptides on the lipid membrane surface, enhancing the disruption of membranes [176,177]. In addition, it has been shown that the presence of calcium ions improves binding of A\(\beta\)-peptide monomers to lipids [178] and blocks their insertion into membranes [179].
The negatively charged lipids, PS lipids in particular, are widespread in neuronal membranes. PS lipids are one of the substantial players in apoptosis [180]; they serve as signal molecules that provide recognition and uptake of apoptotic cells by phagocytes [181]. Emergence of strong electrostatic interactions between positively charged A\(\beta\) peptide's moieties and negatively charged lipids has been reported repeatedly [63,64,68,70,182]. At the same time, charged lipids were shown to prevent the A\(\beta\) peptides being released from the membrane [183] or to assist the peptide aggregation resulting in A\(\beta\) oligomerization and fibrilization [184,185]. Hence, both processes may be relevant for the development of preclinical AD stage.
The role of cholesterol and melatonin, as well as the role of different ions and membrane lipid composition, is of a great importance in understanding molecular mechanisms of associated diseases. Specifically, such molecular additives may potentially serve as a tool aimed at regulating A\(\beta\)(25–35)-triggered membrane destabilization, which leads to the membrane morphological reorganizations (ULVs–BLSs–SUVs) discussed previously [84,128]. Thus, the control of membrane fluidity through the addition of the various additives may be attempted. Unraveling their impact on the overall structure of membranes with incorporated A\(\beta\)(25–35) molecules, three particular types of effects have been observed. First, the changes of ULV structural parameters were experimentally detected upon addition of cholesterol to the lipid membranes with incorporated A\(\beta\)(25–35) by using SANS [162]. Cholesterol affected the curvature of such membranes so that ULVs were of much larger sizes than those formed by the lipid membranes without cholesterol. The effect has been explained by the increased membrane rigidity induced by cholesterol, similar as in the case of A\(\beta\)(25–35) addition. Second, melatonin molecules and charged PS lipids were demonstrated not to contribute to the structure of membranes with embedded peptides [162,186]: they were shown not to affect significantly either the membrane thickness or the ULV size. Finally, calcium ions in the millimolar concentration range were found to inhibit the effect of A\(\beta\)(25–35) on the elastic properties of lipid membranes resulting in decrease in ULV sizes and corresponding decrease in the membrane rigidity as revealed by SAXS and SANS experiments [87]. The MD simulations detected the deep incorporation of A\(\beta\)(25–35) molecules, which was unaffected by calcium ions addition, in the hydrophobic part of lipid membrane [87]. In addition, the A\(\beta\)(25–35) secondary structure changed partly from random coils to \(\alpha\)-helices upon the addition of calcium ions [87]. However, all the mentioned additives were detected to neither prevent, nor stimulate the A\(\beta\)(25–35)-triggered morphological membrane reorganizations and thus the membrane disintegration driven by A\(\beta\)(25–35) molecules.
6. Summary and outlook
All the effects discussed in this review become important for understanding not only the interactions between lipids and corresponding molecular constituents in cell membranes but, more importantly, for revealing opportunities to control the structural and dynamic properties of lipid membranes. After all, membranes are at a forefront of cell function and dysfunction, defense mechanisms, or disease developments. One example that is a focus of this review is the interactions between A\(\beta\) peptides and lipid membranes at the early stage of Alzheimer's disease. We have gathered together the works that establish the amyloid cytotoxicity observable via the A\(\beta\)-triggered morphological membrane reorganizations between spherical vesicles and flat bicelle-like structures. The geometries of these morphologies and their structural arrangements indicate directly the temporal destabilization of membranes during the process of reorganization. More importantly, this effect becomes instrumental in its further exploitation for studying the possibilities to prevent the membrane destabilization via various biologically active additives. The promising examples that have been reviewed comprise the addition of cholesterol and/or melatonin for controlling the membrane elasto-mechanical characteristics, and introduction of charged lipids into the membrane or ions into surrounding environment for controlling the electrostatic interactions. Despite the local impacts on the membrane structure and/or inner structure of A\(\beta\) peptides, all the additives discussed were found impotent in preventing the A\(\beta\)-triggered morphological membrane reorganizations and thus the membrane destabilization driven by A\(\beta\) peptides.
Acknowledgements
This work has been supported by the JINR topical plan [theme 04-4-1149-2-2021/2028] with additional support for S.K. [grant AYSS-23-402-06] and N.K. [VEGA 1/0305/ 24]. We acknowledge the utilization of the Center for shared facilities of Kazan Federal University, access to the HybriLIT heterogeneous computing platform, Govorun supercomputer, and IBR-2 reactor.
Author contributions
S.K.: Writing — original draft preparation, Visualization, Writing — review & editing; O.I.: Writing — review & editing; T.M.: Writing — review & editing; D.B.: Visualization, Writing — review & editing; E.D.: Visualization, Writing — review & editing; E.E.: Writing — review & editing; A.K.: Writing — review & editing; N.K.: Conceptualization, Writing — review & editing.
Conflicts of Interest
The authors declare no conflicts of interest.