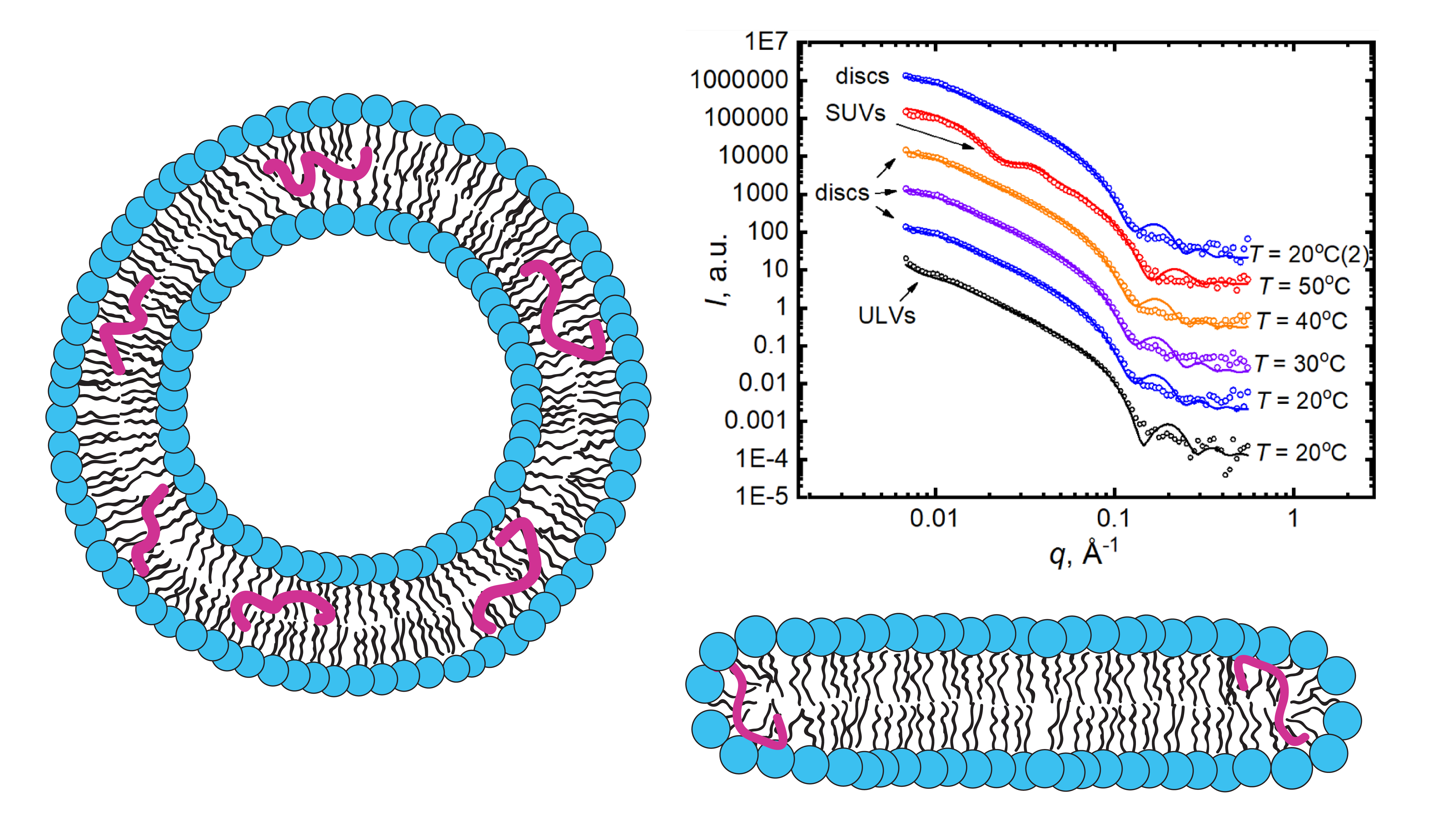
The amyloid-beta peptide (Aβ peptide) is proposed to play a central role in the onset of Alzheimer’s disease (AD). The pathology is associated with the fast accumulation of neurotoxic amyloid aggregates in brain tissues, though the fundamentals of the disease’s progression remain unsolved. It is noted that the preclinical stage of AD may play a crucial role in its further irreversible development. Namely, interactions between lipid membranes and Aβ-peptide molecules incorporated therein at relatively low concentrations should be under a close attention. In this review, we discuss recent works devoted to studying the lipid peptide interactions with a specific focus on the lipid membrane reorganizations caused by Aβ (25–35) peptide in the preclinical AD mimicking conditions. The interactions observed are believed to be important in understanding the mechanisms of the Aβ-peptide destructive effects on lipid membranes and the corresponding onset of the disease. The methods of applied nuclear physics have proven remarkably relevant in such research. The scattering methods provided instrumental information on a level of supramolecular assemblies, while spectrometry allowed obtaining information on the molecular level. Finally, molecular dynamics simulations provided details unachievable by experimental approaches, though the validation role of the latter cannot be undermined. Altogether, the recent advances in research results prove these complementary approaches the most appropriate for tackling the complex issues of biomembrane interactions.
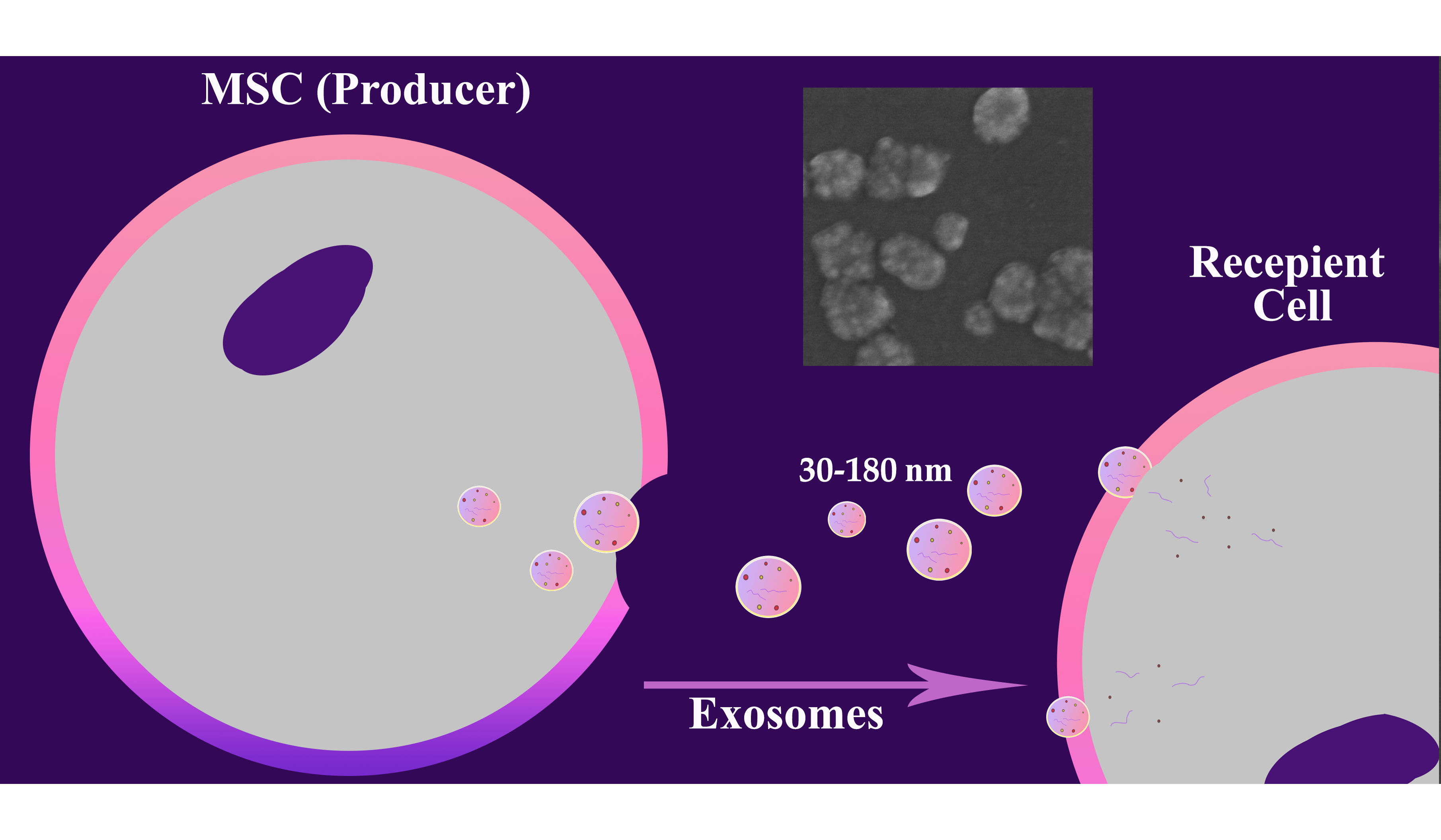
This study provides a comparative analysis of various components of mesenchymal stem cells (MSC) conditioned media (CM) obtained using serum-containing and serum-free culture methods, revealing significant differences in their composition and potential clinical applicability. Serum-containing CM exhibits significantly higher levels of total protein, non-vesicular RNA, exosomes, and nanoparticles compared to serum-free CM, reflecting the contribution of both the MSC secretome and residual fetal bovine serum components. Ultrafiltration-based fractionation (0.2 µm–50 kDa) allows the isolation of fraction enriched in exosomes and proteins, preserving the functionally significant components of the MSC secretome. This strategy effectively captures small vesicles and mid-sized proteins while excluding larger or smaller biomolecules, enhancing utility for targeted analyses. The presented data underscore the need for context-driven CM selection and provide information for choosing the optimal strategy for obtaining the MSC secretome balancing yield, purity, and regulatory demands in MSC research and therapy.